Saturday, July 10, 2010
Orbital Theories
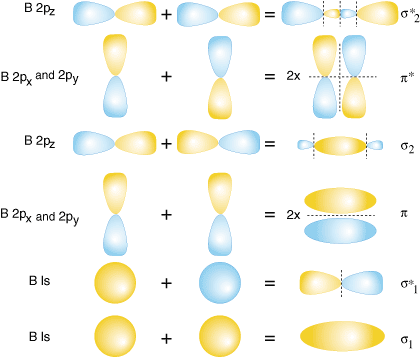
-Molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but treated as moving under the influence of the nuclei in the whole molecule.
-In this theory, each molecule has a set of molecular orbitals, in which it is assumed that the molecular orbital wave function ψf may be written as a simple weighted sum of the n constituent atomic orbitals χi , according to the following equation: 

-The cij coefficients may be determined numerically by substitution of this equation into the Schrödinger equation and application of the variational principle. This method is called the linear combination of atomic orbitals approximation and is used in computational chemistry. An additional unitary transformation can be applied on the system to accelerate the convergence in some computational schemes.

9:23 PM
